co2-3 molecular geometry|9.2: VSEPR : iloilo 2 days ago — CO2 has a linear shape. The bond angle of CO2 is 180°. The molecular geometry of any compound can be determined by the VSEPR theory. The VSEPR chart is attached below, which will give us an idea . Discover and play now over 7 of the best Flash games on Kongregate such as , and many more! To enhance your user experience, support technical features, and personalize content and ads, this site uses cookies.
PH0 · Properties of the Carbon Dioxide Molecule
PH1 · Molecular Geometry Of CO2: How Lewis Structure
PH2 · CO3 2
PH3 · CO2 Molecular Geometry and Bond Angles (Carbon Dioxide)
PH4 · CO2 Lewis Structure, Molecular Geometry, Molar
PH5 · CO2 Lewis Structure, Molecular Geometry and Hybridization
PH6 · CO2 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagr
PH7 · CO2 Lewis Structure, Hybridization, Molecular
PH8 · 9.2: VSEPR
PH9 · 8.6: Molecular Geometries
PH10 · 5.2: Molecular Shape
CO2 has a linear shape. The bond angle of CO2 is 180°. The molecular geometry of any compound can be determined by the VSEPR theory. The VSEPR chart is attached below, which will give us an idea about this. So from the above chart, it’s clear that CO2 is an AX2 type molecule, where X= bonded atom. Thus the molecular geometry of .
co2-3 molecular geometry*******Hul 6, 2013 — A quick explanation of the molecular geometry of CO2 including a description of the CO2 bond angles. We can see that there are only two atoms attached to the central Carbon (C) atom .
Assign an AX m E n designation; then identify the LP–LP, LP–BP, or BP–BP interactions and predict deviations from ideal bond angles. Describe the molecular geometry. We .
Two regions of electron density around a central atom in a molecule form a linear geometry; three regions form a trigonal planar geometry; four regions form a tetrahedral geometry; five regions form a trigonal .2 days ago — CO2 has a linear shape. The bond angle of CO2 is 180°. The molecular geometry of any compound can be determined by the VSEPR theory. The VSEPR chart is attached below, which will give us an idea .There is a three step approach to determining the geometry of a molecule. Determine the Lewis dot structure of the compound. Determine the Electron geometry from the Lewis dot structure. Determine the molecular .
Ene 5, 2021 — In its electronic ground state, the carbon dioxide molecule has a linear geometry (Fig. 7.1) and belongs to the point group D ∞h. Both C-O bonds are .Dis 12, 2020 — CO2 Molecular Geometry & Shape. In a CO2 molecule, the carbon atom is in the center double bonded with two oxygen atoms by each side. Both oxygen atoms have two lone pairs of nonbonding .What is the molecular geometry of CO 2? CO 2 has a molecular geometry. According to the VSEPR theory, the shape of a molecule can be predicted by considering the electron pair repulsion in the valence .
1 day ago — In carbonate ion, among the two elements, Carbon has an electronegativity value of 2.55 whereas Oxygen has a high value of 3.44. As per common procedure, the one with the least electronegativity value .Molecular geometry is a way of describing the shapes of molecules. It applies a theory called VESPR for short. VESPR stands for valence shell electron pair repulsion. This theory basically says that bonding and non .
Ago 20, 2021 — The arrangement of three regions of high electron density gives a trigonal planar electron-pair geometry. The B–Cl bonds lie in a plane with 120° angles between them. BCl 3 also has a trigonal planar molecular structure. The electron-pair geometry and molecular structure of BCl 3 are both trigonal planar. Note that the VSEPR geometry .Molecular geometry, on the other hand, depends on not only on the number of electron groups, but also on the number of lone pairs. . Inverted geometries at carbon Kenneth B. Wiberg Acc. Chem. Res.; 1984 "Molecular Geometries." Chemistry Foundations and Applications. Volume 3. Farmington, MI:Lagowski, J.J., 2004.Example \(\PageIndex{1}\): Predicting Electron-pair Geometry and Molecular Structure. Predict the electron-pair geometry and molecular structure for each of the following: carbon dioxide, CO 2, a molecule produced by the combustion of fossil fuels; boron trichloride, BCl 3, an important industrial chemical; Solution (a) We write the Lewis .

Hun 19, 2023 — We see from Figure 6.3.3 that the molecular geometry of CO 3 2 . Use Figure 5.1.3 to determine the molecular geometry around each carbon atom and then deduce the structure of the molecule as a whole. Solution: Because the carbon atom on the left is bonded to four other atoms, we know that it is approximately tetrahedral. The .
Ago 14, 2020 — (b) The dotted lines illustrate that the hydrogens form a tetrahedron about the carbon atom. We conclude that molecular geometry is determined by minimizing the mutual repulsion of the valence shell electron pairs. As such, this model of molecular geometry is often referred to as the valence shell electron pair repulsion (VSEPR) theory.
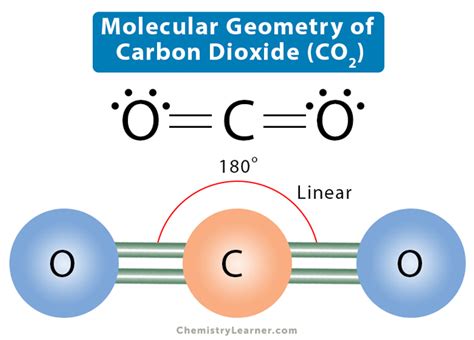
The carbon-oxygen ratio in a CO 2 molecule is 1:2. Two double bonds connect the carbon and oxygen atoms in the Lewis structure. Two oxygen atoms are present at the terminals, where they share electrons and form bonds with the central carbon atom. . CO 2 molecular geometry is based on a linear arrangement. The presence of a sigma bond .
Molecular geometry is the name of the geometry used to describe the shape of a molecule. The electron-pair geometry provides a guide to the bond angles of between a terminal-central-terminal atom in a compound. . The shape we see is the only possible shape for a central carbon atom with four bonds. This geometry is a direct result of the .co2-3 molecular geometryOkt 11, 2022 — The steric number of central carbon in CO 3 2- is 3 so it has sp 2 hybridization. The [CO 3] 2- bond angle. The bonded atoms in [CO 3] 2-ion form ideal bond angles as expected in a symmetrical trigonal planar molecule. . What are the molecular geometries of BeCl 2, [CO 3] 2-, CH 4, and [H 3 O] +?1 day ago — Here, activated porous carbon acts as the catalyst. . Molecular Geometry. It is the 3-dimensional atomic arrangement that gives us the orientation of atomic elements inside a molecular structural .
co2-3 molecular geometry 9.2: VSEPR Okt 28, 2020 — CH3OH Molecular Geometry. Now that we know the Lewis structure of CH3OH, it is easy to depict the compound’s molecular geometry. While drawing the Lewis structure for CH3OH, you will notice .2 days ago — CO2 has a linear shape. The bond angle of CO2 is 180°. The molecular geometry of any compound can be determined by the VSEPR theory. The VSEPR chart is attached below, which will give us an idea .
Okt 10, 2023 — Why the molecular geometry of CO2 is linear? The molecular geometry of CO2 is linear. Because the carbon (C) central atom has no lone pair and is attached to the two oxygen (O) atoms. So, there are two regions of electron density around the carbon central atom, based on VSEPR theory, it will acquire linear molecular geometry.Molecular geometries (linear, trigonal, tetrahedral, trigonal bipyramidal, and octahedral) are determined by the VSEPR theory. . The central carbon atom is still joined to two other atoms. The electron clouds that connect the two oxygen atoms are 180° apart. Trigonal Planar. Molecules with the trigonal planar shape are triangular and in one .Ene 5, 2021 — 7.1.1 The Ground State of Carbon Dioxide and Its Geometry. In its electronic ground state, the carbon dioxide molecule has a linear geometry (Fig. 7.1) and belongs to the point group D ∞h. Both C-O bonds are equivalent with an equilibrium distance equal to 116.00 pm, as established by electron diffraction .9.2: VSEPR Figure \(\PageIndex{3}\): Molecular orbital diagram for carbon dioxide. (CC-BY-NC-SA; Kathryn Haas) The SALCs on the right side of the diagram above are constructed from groups of either the \(2s\) atomic orbitals of oxygen, or the \(2p\) atomic orbitals of oxygen. Each SALC will combine with the atomic orbitals of carbon that have compatible .
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. . carbon dioxide and nitric oxide have a linear molecular shape. Trigonal planar: Molecules with the trigonal planar shape are somewhat triangular and in one plane (flat). Consequently, the bond angles are set at 120°. For example, boron trifluoride.
Ano-ano ang Mga P’wedeng Gamot sa Sakit sa Puso? May iba’t-ibang uri ng gamot na maaaring gamitin sa pangangalaga at pagkontrol ng sakit sa puso. Mula sa mga over-the-counter na gamot papunta sa mga natural o herbal na paraan, narito ang ilan sa kanila: Antiplatelet Agents
co2-3 molecular geometry|9.2: VSEPR